COVID safety notes
- the researcher's and the participant's safety should both be taken into consideration
- researchers should always wear a mask while conducting studies
- protocols will be explained to shorten the exposure between researchers and participants
Being prepared
Paperwork to have ready:
- Consent
- Demographics
- EEG log sheet
- Papers for written tasks
- Receipt form and compensation for paid studies
Important Supplies:
- Measuring tape
- Grease pencil
- Hair clips
- 2 full, pre-filled syringes and extra gel
- an extra syringe needle
- Wooden dowels
- Paper towels
- Electrode sticky discs
- Electrodes VEOL, VEOU, HEOL, HEOR, and nose reference (long wire)
- Missing or broken electrodes can be substituted with another of a different label as long as it is applied and connected in the proper location
First Steps:
- Prepare electrodes VEOx, HEOx, and the nose reference
- Remove the sticky disks from the sheet (it helps to poke the center hole out first) and stick to electrode, centering it as best as possible
- Fill electrode with gel as completely as possible without mounding the gel (doing this early allows it to dry and makes it more effective later)
- Turn on electronics in order
- Turn on Amy (mouse and keyboard switcher back left on desk switches between Amy and Hypnotoad)
- Turn on Hypnotoad. Once on, open ISCAN
- Turn on Kif. Once on, move to appropriate directory, open: PTB
Put your phone on silent
Setting Up the Pariticipant
When the participant arrives, ask them to remove all electronics (i.e. phones, watches, earbuds, etc.) and store them in a safe space.
Give the participant the informed consent sheet and any other paperwork that needs to be done at this time.
- Let the participant know this will be a long study, and that if they need to go to the bathroom, they should do so at this point
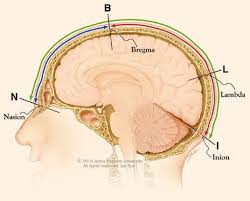
Using the tape measure, have the participant hold the start of it in the center of their forehead, just above the eyebrows. Make sure that the bottom of the tape measure touches the tops of the ears and the inion. Write down the total number in cm (this measurement will tell you if they require a S, M, or L cap).
Have the participant hold the tape measure against their nasion (an indent just below the eyebrows) and go over the top, center of their head until you reach the inion (a bump at the lower, back part of the skull). Take 10% of that number, then measure that distance on the participant's forehead and mark it with a grease pen (this will be a reference for where the front of the cap should be placed).
Guide the participant into the EEG study room and check the EEG cap log to make sure to use the least recently used cap in that size.
Have the participant brush through their hair with a disposable comb and clean off their face with a towelette (specifically on their nose and around their eyes, as electrodes will be placed there).
With the proper cap, have the participant sit still. Align the front of the cap with the grease mark using your thumbs along the front edge to hold it in place, and assure that the central electrodes are lined up with the center of their head. Pull the cap over their head using the rest of your fingers.
- if this process is done properly, the back of the cap should fall on the inion, the ear holes should be comfortable for the participant, and the strap should fit snuggly under their chin
- While the cap is difficult to adjust once its on the participant's head, if there is a need to move the cap, don't tug on a specific section, but try to move the entire cap
Have the subject hold the cap connector
Remove the backside of the sticky disc and apply each electrode
- HEOR – just outside of right eye, connector hangs near right ear
- HEOL – just outside of left eye, connector hangs near left ear
- VEOU – just above left eyebrow, connector hangs near left temple
- VEOL – just below left eye, at top of cheekbone, connector near left temple
- Nose reference –tip of nose, connects at bottom rear of cap
- Be sure to audibly say "boop" as you stick it to their nose
- Run wire along side of the cap and secure it out of the way with a mini hair-clip.
Have the participant face the monitor, and adjust the chair and the chin rest so that they are in a comfortable position. Be sure that it is a position they can stay in comfortably for an extended period of time, as we want them to be comfortable during the experiment.
When they are comfortable, connect the cap connector to amplifier and turn the amplifier on.
Testing and reducing impedance
On subject monitor, hit: source, down arrow to DVI, Menu. This will connect display to Amy so that impedances can be checked from subject room.
Prepare for new acquisition:Open Curry 8oDUnder database pane
- click experiment name, (verify that current subject # has not already been used)
- Rclick experiment name
- select ‘add new subject’
- Rclick new subject
- select 'rename' to change the name to ‘experimentname_sub_XXX'
- Rclick subject just created
- select ‘add new study’
- Rclick new study
- select rename to change the name to ‘as Session_01’
- Place cursor over session_1
- click the red circled arrow that appears to open acquisition pane
Under acquisition pane, (amplifier control tab already open) verify amplifier = SynAmps2/RT, configuration = RefRemyourstudyconfig, sample rate = 1kHz.
- Under the options tab, lower set page size to = 2and scaling = 10.
Back to Amplifier control tab
- click ‘start amplifier’
- click the symbol to start the impedance test.
You should see the impedance chart on the monitor. They will all show high impedance at this point. Only the electrode sites to be used in the current study will appear.
Explain the syringes to the subject, show them that they are not sharp. Tell them that we will be filling each electrode with a small amount of gel and in order to establish a good connection, will be wiggling the end of the syringes or a wooden swap in the electrode to move hair and allow gel to contact the scalp. We will be swirling firmly, however if it becomes uncomfortable, the subject should let us know!
Start by putting gel in the Ground electrode and another electrode for example CZ (select an easy one from the screen’s chart, usually one relatively unobstructed by hair). These plus the references, which should already have decent connection since they were resting before the experiment began, are important in establishing a good baseline. Once the third electrode (CZ) has been gelled, it’s impedance should be lower.
Try to achieve <5 kΩ on the third electrode by continuing the swirling action. If it won't go lower than 5 kΩ, the ground and references should be tried again. It is important to establish these 3 electrodes below 5 kΩ before moving on to the others.
Make one quick pass over each other electrode, filling with gel and swirling just enough to see its impedance jump lower, then move on to the next. Once all electrodes have been gelled, return to them and continue the swirling motion.
if <5 kΩ can be achieved quickly that is fine but on the second pass, shoot for 10 kΩ.
- on the third pass, return to any electrodes above 5 kΩ and continue to work on them. Once all electrodes are below 5 kΩ, double check the impedances on the screen.
Switch subject monitor back to HDMI (Kif), and begin the experiment.
If eye tracking is used in the study, refer to StandardEyeTrackingProtocol
EEG Data Collection Checklist
Practice Run
- Run script on Kif for experiment task “XXXXXXX”
- Verify refresh rate at 60Hz, hit any key to continue.
Click Yes for practice run, enter 3-digit subject number, verify outfile name –if run number is incorrect, click no and enter manually –make note on log
- Click yes to eyetracking and EEG data collection.
- On Amy, under amplifier control tab, click the red record button
Verify recording before starting task!
- Hit trigger key to begin task (*)
Note: As soon as the task ends, click the record button again on Amy to stop recording.
First Experimental Run
- Close door before starting actual runs
- Run script on Kif for experiment task “XXXXXXX”
- Verify refresh rate at 60Hz, hit any key to continue
Click No for practice run, enter 3-digit subject number, verify outfile name –if run number is incorrect, click no and enter manually –make note on log
- Click yes to eyetracking and EEG data collection
- On Amy, under amplifier control tab, make sure that you have stopped the previous recording and have stopped the amplifier after the last run
- Start the amplifier up again and click the red record button
Verify recording before starting task!
- On each run, when you click record again, it will automatically save the run under a new acquisition.
- Hit trigger key to begin task (*)
Note: As soon as the task ends; click the record button again on Amy to stop recording
Second Run Onward
- Prior to recording or running task script, click the button on Amy in the amplifier pane and check all impedances. If any are above 5 kΩ, add gel and correct to get below 5 kΩ. Note any adjustments in the log (which electrodes and what kΩ they were up to)
- Before every run, be sure that the door is closed
- Click the button to close the impedance screen and continue with the task
- Hit the task trigger key to begin the task run(*)
- Keep an eye on behavior and EEG signal throughout the run, making notes of anything unusual on the log form.
Cleaning Up
After stopping recording on last session, also click on stop amplifier. Carefully disconnect cap from amplifier, hand connector end to subject, and lead back out to the setup area. Remove chin strap. Disconnect face electrodes carefully, slowly peel sticky discs away from skin. Gently remove the EEG cap and place in sink ensuring that connector is not in danger of getting wet at any point. Offer participant another towel, hair dryer, bathroom break, anything else they need before wrapping up paperwork and debriefing. When participant is ready, do remaining forms: Derryberry, vocab. LTM?? Give participant payment and have them fill out receipt form.Read debriefing script; check if participant has any questions. Thank participant for coming in and show them out.
Shut down computers in the following order:
- Close Curry 8 and shut down Amy.
- Close ISCAN and shut down Hypnotoad.
- Quit PTB, shut down Kif.
Needle end of syringes should be disposed of in the biohazard container. Leave remaining gel in syringe, but pull plunger back a small bit to pull gel back from tip.
Throw out any used paper towels, swabs, and other disposable items used in the study
Put any used towels in the laundry basket.
Put measuring tape in the disinfectant bucket.
Remove the reference electrodes from the cap and throw away the sticky disks. Using the water pick, on setting 4, spray the electrode and make sure there is no remaining gel. Spray through the hole as well to make sure its clean. Then, place the reference electrodes in the disinfectant bucket.
Keeping the cap connector away from the water, gently rinse the cap in sink, try to keep its shape without stretching the material.
Turn cap inside out and using the water pick, on setting 4, spray each electrode cup to remove any gel, make sure to move the stream through the hole in the electrode.
- Spray each electrode moving from electrode to electrode in a pattern to ensure none are missed.
the water pick uses distilled water, so if you run out, simply get more from the water distiller
Rinse the cap under the faucet once more to remove stray gel. Inspect each electrode and make sure no gel remains! Also clean off any gel that remains on the fabric of the cap.
Remove the measuring tape and electrodes from the disinfectant, and lay them out on paper towels to dry.
Place the cap in the disinfectant bucket for 2 minutes, again keeping the cap connector away from the liquid. Then remove and rinse well.
Cover the foam head with a few sheets of paper towels and place cap on head. Position the cap so that it sits loosely and not bunched up anywhere.
Make sure to log any technical irregularities with the net (bad electrodes, etc.) in the collaborators’ EEG net usage logbook.
If more distilled water is needed (basically if there is any less than a full supply --so probably almost always), distill another batch
- Transfer any already-distilled water from the glass distiller jar to a gallon jug (using the funnel that should be in the area).
- Rinse out the metal distiller reservoir with tap water, then fill it with tap water. Make sure it is closed tightly.
- Place the metal distiller reservoir and the glass distiller jar in their appropriate locations. Make sure the reservoir is snug and the jar is in exactly the right spot.
- Switch the distiller on. If possible, try to remember to check back in a few hours.